In the field of sterile packaging manufacturing, sterilization flat rolls are becoming a vital link between cleanroom compliance, sterilization performance, and sustainable packaging practices. Widely used in packaging for medical devices, diagnostic kits, and biopharmaceuticals, these materials and their associated systems are undergoing multidimensional upgrades in response to evolving end-use requirements. New industry standards are now emerging, particularly around clean manufacturing protocols, full-process quality visualization, and the adoption of eco-conscious materials.
Today, high-standard sterilization flat rolls are typically produced in ISO Class 4–5 cleanroom environments. Combined with full-process batch traceability, each roll is linked to complete records of manufacturing parameters and inspection results from production to storage. This traceable framework not only enhances product sterility assurance but also supports downstream sterilization validation and regulatory compliance audits.
Material compatibility is another key focus. Next-generation flat roll packaging is designed to work seamlessly with major sterilization methods such as steam, ethylene oxide (EO), and low-temperature plasma. These materials maintain sealing strength and structural integrity across diverse sterilization environments, allowing users to streamline workflows without the need to switch packaging types for each application.
Sustainability is also a growing priority. An increasing number of manufacturers are adopting solvent-free bonding processes, low-migration inks, and recyclable base films to reduce the carbon footprint without compromising performance. Some structures now incorporate bio-based plastic content to further align with global medical packaging sustainability initiatives.
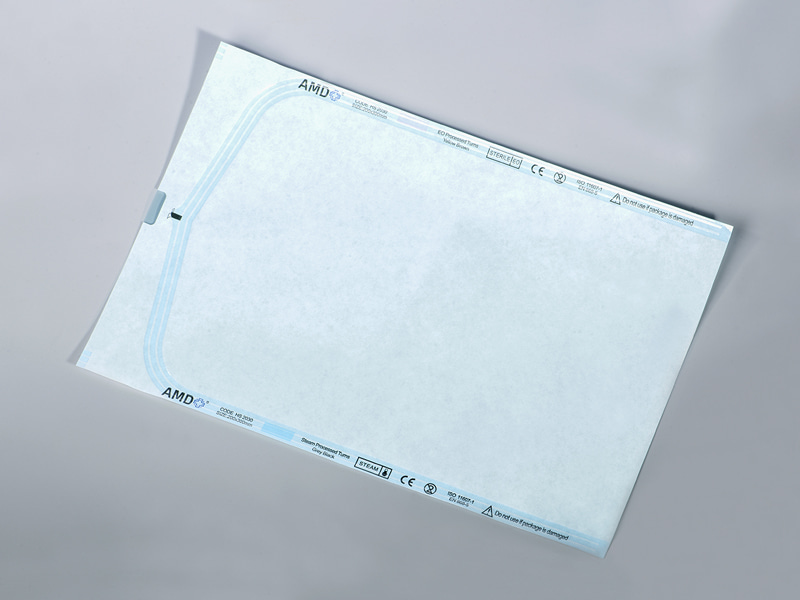
Visual quality control—particularly seal visibility—plays a direct role in operational efficiency. Modern sterilization flat rolls often use high-clarity multilayer films that allow operators to visually inspect the contents and heat-sealed areas with ease. This design minimizes the risk of compromised seals and product loss, and is particularly well-suited for automated filling and high-speed packaging lines where in-line inspection is critical.
On the equipment side, compatibility is now a standard requirement. Flat rolls are engineered with consistent tensile strength, heat-sealing temperature ranges, and tension parameters, allowing flexible integration across form-fill-seal (FFS) systems, automatic cutting machines, and cleanroom robotic packaging units. Systemization, standardization, and automation are becoming essential capabilities for advancing clean packaging performance and throughput.
Product Feature Overview Table
| Technical Dimension | Function Description |
| Cleanroom Manufacturing | ISO-compliant environments with particle and microbial control |
| Batch Traceability | Unique identifier per roll with full production and testing documentation |
| Sterilization Compatibility | Supports Steam, EO, and Plasma sterilization processes |
| Eco-Friendly Process | Uses recyclable base films, low-ink print surfaces, and solvent-free bonding |
| Visual QC | Transparent film enables visual inspection of contents and seal integrity |
| Multi-System Integration |
Compatible with sealing machines, filling systems, and cleanroom equipment |
In today’s increasingly complex sterile packaging landscape, sterilization flat rolls are no longer just containment materials. They are key enablers of regulatory compliance, operational efficiency, and environmentally responsible manufacturing. Medical and laboratory producers are now rapidly integrating these products into their workflows to establish packaging solutions that balance safety, sustainability, and performance. In the future, sterilization flat rolls will continue evolving—not merely as packaging layers, but as integral components of end-to-end sterility assurance systems.

 English
English Français
Français Deutsch
Deutsch Nederlands
Nederlands











 ‘s-Gravenweg 542, 3065SG RotterdamThe Netherlands
‘s-Gravenweg 542, 3065SG RotterdamThe Netherlands
 +31 (0)10 254 28 08
+31 (0)10 254 28 08







