As global standards for sterile packaging continue to rise, Tyvek breathing covers are gaining strategic importance across cleanroom-intensive industries. Known for their excellence in cleanliness, barrier protection, sterilization compatibility, and multi-use durability, these covers are now widely adopted in pharmaceuticals, medical device production, biotechnology, and laboratory operations. Recently, their role in protecting cleanroom equipment components has been further emphasized due to increased sustainability demands and tighter contamination control standards.
Tyvek breathing covers are primarily manufactured in ISO Class 4 cleanrooms and comply with internationally recognized medical packaging standards such as ISO 13485 and EN 868. Each production batch is assigned a traceable identifier and is subjected to both particle and sterility inspections. This manufacturing rigor ensures a stable microbial barrier before the product ever enters a user environment.
Production & Certification Specifications
| Specification | Details |
|
Cleanroom Classification |
ISO Class 4 |
|
Quality Compliance |
Meets ISO 13485 and EN 868 |
| Batch Traceability |
Unique lot identification and full records |
| Particle Monitoring |
Regular checks as per sterile packaging norms |
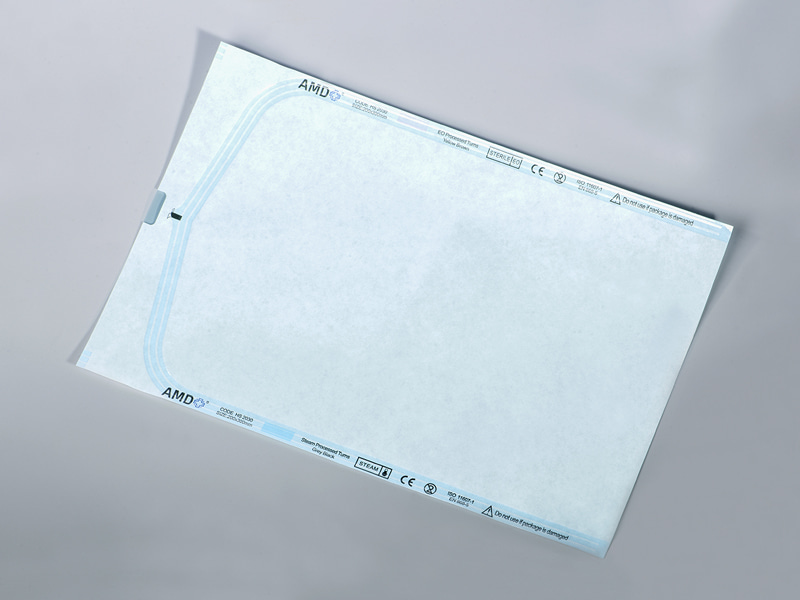
The cover's structure features a lightweight porous Tyvek layer on one side and an optional barrier film on the other. This design ensures sterilants like VHP (vaporized hydrogen peroxide) or EO (ethylene oxide) can pass through the material during sterilization, while liquids, particulates, and microbes are blocked. The fibrous strength of Tyvek also provides resistance to tearing, preserving package integrity under mechanical stress.
Structural and Functional Performance
| Feature | Description |
| Breathability | Allows sterilant penetration, prevents condensation |
| Barrier Performance | Blocks liquids, microbes, and particulates |
| Tear Resistance | High tensile strength maintains integrity during handling |
| Application Scope | Suitable for hoods, trays, valves, tubing, and critical covers |
In practical use, Tyvek breathing covers are compatible with multiple cycles of high-temperature steam sterilization and do not require modifications to existing autoclave systems. Their reusability adds environmental value by reducing the need for frequent replacements.
Sterilization Compatibility
| Aspect | Details |
| Supported Methods | Steam, EO, VHP, and other major sterilization formats |
| Thermal Stability | Maintains structure and performance in moist heat conditions |
| Reusability | Multiple cycles with consistent barrier performance |
| System Integration | Compatible with current sterilization and packaging setups |
Driven by both clean manufacturing mandates and green regulation trends, manufacturers are increasingly standardizing Tyvek covers not only for product packaging but also for internal zone protection—such as automation interface shielding, interim storage wrapping, and controlled transit applications. Their low particle generation and compatibility with critical surfaces make them ideal for high-standard environments.
In summary, Tyvek breathing covers are evolving from passive packaging elements into active components of advanced contamination control systems. As global healthcare logistics and sustainability regulations accelerate, their value and application scope will continue to grow.
Summary of Key Advantages
| Dimension | Performance of Tyvek Breathing Covers |
| Sterility Assurance | Produced in clean environments with traceable batch control |
| Mechanical Strength | Durable and tear-resistant even in rigorous operations |
| Sterilization Fit | Compatible with moist heat and gas sterilization |
| Environmental Benefit | Reusable across cycles, reducing material turnover |
| Cross-Industry Use | Applicable to pharma, medtech, biotech, and laboratory settings |
For detailed product specifications, comprehensive certification documentation, or expert guidance on integrating Tyvek breathing covers into your cleanroom or sterilization workflow, please reach out to the official technical support team for tailored assistance based on your specific operational needs.

 English
English Français
Français Deutsch
Deutsch Nederlands
Nederlands











 ‘s-Gravenweg 542, 3065SG RotterdamThe Netherlands
‘s-Gravenweg 542, 3065SG RotterdamThe Netherlands
 +31 (0)10 254 28 08
+31 (0)10 254 28 08







