A sterilization reel (or sterile roll) is a sterilizable packaging material designed specifically for the medical, laboratory, or food industries. It is typically supplied in roll form and is used to wrap instruments, consumables, and other items. After high-temperature and high-pressure sterilization (such as steam sterilization), it maintains sterility until use.
Content
1. Function of Sterilization Reels
Material: Common materials include medical-grade kraft paper, Tyvek®, and plastic film laminates.
Format: Supplied in rolls that can be cut to desired lengths and then sealed and sterilized after wrapping the item.
Sterilization Compatibility: Withstands high-temperature and high-pressure sterilization (121°C–134°C), ethylene oxide (EO), or radiation sterilization.
Barrier Protection: After sterilization, it blocks microorganisms and maintains the sterility of the contents.
Storage Convenience: The roll format saves space and can be cut as needed.
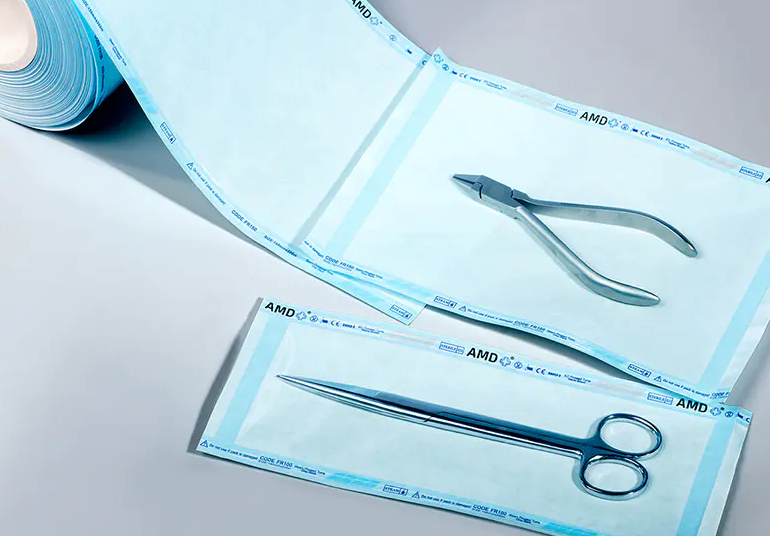
2. Sterilization Reels vs. Conventional Packaging Materials
| Comparison Items | Sterilization Reels | Common Wrapping Paper/Film |
| Sterilization Resistance | Designed for high temperature, high pressure, EO, and radiation exposure, no cracking | May melt, deform, or release toxic substances |
| Microbial Barrier | Tested to ASTM F1608 and other standards, preventing bacterial penetration | No certification, high risk of porosity |
| Breathability | Some materials (such as Tyvek®) are breathable but not bacteria-permeable | Often completely sealed or with uneven breathability |
3. Usage Example (Using Surgical Instrument Sterilization)
Cutting: Cut the reel according to the size of the instrument, leaving enough space for wrapping.
Wrapping: Fold the reel in two layers to prevent sharp edges of the instrument from puncturing the material.
Sealing: Seal with self-adhesive tape or a heat sealer. Label the reel with the sterilization date and contents.
Sterilization: Place in an autoclave (121°C, 20 minutes).
Storage: Store in a dry environment. The shelf life depends on the material (e.g., Tyvek® can last up to 1 year).
4. Replacement Standards for Sterilization Reels
(1). Physical damage
Manifestations: tears, perforations, edge delamination (especially at folds).
Risk: Direct microbial intrusion and failure of the sterile barrier.
Test method: Translucent inspection (hold the reel up to the light, no translucent spots).
(2). Wet pack or contamination
Manifestations: Water stains, blood stains or chemical residues on the packaging after sterilization.
Handling: Replace immediately and trace the sterilization process (such as insufficient drying time).
(3). Sealing failure
Manifestations: The tape/heat seal edge lifts and loses self-adhesion (such as after repeated opening and closing).
Standard: Replace when the seal width is less than 6mm or the bonding area is less than 80%.
(4). Material aging
Manifestations: Kraft paper reels become brittle and powdery. Tyvek® reels turn yellow and their flexibility decreases.
Accelerated aging conditions: High temperature (>40°C) or ultraviolet exposure.
(5) Exceeding the limit for sterilization times
Upper limit: Repeated sterilization of the same roll material ≤ 3 times (crepe paper) or ≤ 5 times (Tyvek®).

 English
English Français
Français Deutsch
Deutsch Nederlands
Nederlands













 ‘s-Gravenweg 542, 3065SG RotterdamThe Netherlands
‘s-Gravenweg 542, 3065SG RotterdamThe Netherlands
 +31 (0)10 254 28 08
+31 (0)10 254 28 08







