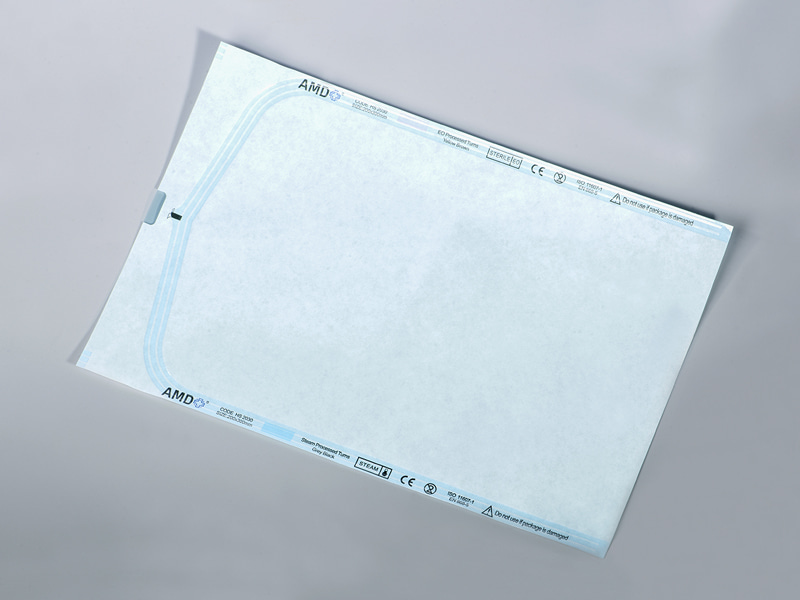
With the growing demand for sterile packaging in the medical device and pharmaceutical industries, Sterilization Gusseted Roll has gradually become a key packaging material due to its flexible design, contamination resistance, and high durability. This article provides an in-depth analysis of its material properties, application advantages, operational procedures, and storage requirements, highlighting its significance and development trends in the industry.
Content
- 1 1. Material Composition and Characteristics
- 2 2. Advantages of Gusseted Design and Contamination Resistance
- 3 3. Usage and Sterilization Compatibility
- 4 4. Contamination Resistance and Durability
- 5 5. Storage and Transport Convenience
- 6 6. Safety and Long-Term Use
- 7 7. Reasons to Choose Sterilization Gusseted Roll
1. Material Composition and Characteristics
Gusseted Roll is typically made of multi-layer composite materials, including non-woven fabric, polypropylene (PP), polyester (PET), and certain high-temperature resistant films. Some products also offer environmentally friendly or biodegradable options to support green medical packaging.
| Material Type | Performance Features | Application Advantages |
| Non-woven fabric | Flexible, breathable | Can wrap irregularly shaped instruments and ensure sterilization gas penetration |
| Polypropylene (PP) film | High-temperature and chemical resistance | Suitable for steam and ethylene oxide (EO) sterilization |
| Polyester (PET) film | High strength, tear-resistant | Prevents punctures and contamination |
| Biodegradable/eco-friendly materials | Biodegradable, low environmental impact | Supports sustainable medical packaging needs |
2. Advantages of Gusseted Design and Contamination Resistance
The Gusseted (accordion-fold) structure adds fold layers on both sides of the roll, enhancing packaging expandability and wrapping capacity. Its core advantages include:
| Feature | Description |
| Increased packaging capacity | Fold layers expand to accommodate instruments of different sizes, reducing material waste |
| Enhanced sealing | Heat-sealing or self-adhesive design ensures edges are completely closed, providing a reliable sterile barrier |
| Contamination resistance | Effective barrier against bacteria, dust, and moisture, verified through microbial penetration tests (e.g., ASTM F1608) |
Additionally, the Gusseted design helps minimize wrinkles during packaging, reducing the risk of seal failure caused by improper folding.
3. Usage and Sterilization Compatibility
(1) Cutting and Sealing
Cutting method: Can be trimmed as needed to fit instruments of various sizes.
Sealing method: Recommended use of heat sealing machines or self-adhesive sealing to ensure complete edge closure.
(2) Sterilization Compatibility
Similar to Sterilization Flat Roll, Sterilization Gusseted Roll supports multiple sterilization methods, including:
Steam sterilization: Resistant to high temperature and pressure without deformation or degradation.
Ethylene oxide (EO) sterilization: Good breathability ensures sterilant penetration throughout the material.
After sterilization, sterility should be verified using seal integrity tests or biological indicators.
4. Contamination Resistance and Durability
Gusseted Roll features a multi-layer composite structure that provides contamination resistance while offering tear and puncture resistance. Its flexible materials can conform to various instrument surfaces and ensure sterilant gases penetrate effectively.
| Performance Indicator | Description |
| Contamination barrier | Blocks dust, bacteria, and microorganisms |
| Tear resistance | Multi-layer composition reduces the risk of accidental damage |
| Puncture resistance | Maintains the integrity of the sterile barrier |
5. Storage and Transport Convenience
The storage requirements for Roll are similar to those of Sterilization Flat Roll, but the roll design enhances efficiency in transport and storage:
Storage environment: Dry, away from light, at room temperature; avoid moisture or temperatures that may affect performance.
Transport convenience: Roll format saves space, making it suitable for bulk transport and warehousing.
6. Safety and Long-Term Use
Gusseted Roll does not produce toxic residues during use, ensuring safe medical instrument packaging. Long-term use does not affect the function or safety of instruments, but each use should follow validation procedures to confirm sterilization effectiveness. Recommended validation methods include:
Physical performance testing: Seal strength, tear resistance, and breathability
Microbial barrier testing: Microbial challenge tests according to relevant standards
Sterilization compatibility testing: Ensures material maintains stable performance after repeated sterilization cycles
7. Reasons to Choose Sterilization Gusseted Roll
In the medical and pharmaceutical packaging sector, this roll material is widely applied due to the following advantages:
1.Reliable sterile barrier: Effectively blocks microorganisms, reducing infection risk
2.High flexibility and increased capacity: Gusseted design accommodates instruments of various sizes
3.Broad sterilization compatibility: Supports multiple sterilization methods, including steam and ethylene oxide (EO)
4.Environmental and biodegradable options: Meets sustainable medical packaging requirements
5.Operational convenience: Compatible with automated packaging equipment, improving workflow efficiency
With the growing demand for efficient sterile packaging, Sterilization Gusseted Roll offers adaptability, safety, and environmental benefits, making it an ideal choice for hospitals, laboratories, and pharmaceutical manufacturers. Future advances in material technology are expected to further optimize performance, driving the medical packaging industry toward more intelligent and sustainable solutions.

 English
English Français
Français Deutsch
Deutsch Nederlands
Nederlands











 ‘s-Gravenweg 542, 3065SG RotterdamThe Netherlands
‘s-Gravenweg 542, 3065SG RotterdamThe Netherlands
 +31 (0)10 254 28 08
+31 (0)10 254 28 08







